Green Molecules and Green Chemistry Labs, Part III

Johnny and Cindy.
It’s been two weeks since my last day at the lab. The time I’ve spent away has given me a different perspective on how I spent my summer.
The things I learned have made me fall in love with chemistry all over again. Solid-state synthesis, gas-phase-synthesis, ionic liquids, phase-transfer catalysis, macrocycle synthesis, these terms have gained new meaning since I had first read about them in a textbook in May (it seems so long ago).
The Aldol condensation is already a green organic chemistry reaction: it has high atom-efficiency and produces water as a byproduct.
 This product was synthesized in the solid-state variation of the reaction, in the absence of a solvent at room temperature. This same technique was used to synthesize chalconoids, a class of chemicals with a variety of medical properties.
This product was synthesized in the solid-state variation of the reaction, in the absence of a solvent at room temperature. This same technique was used to synthesize chalconoids, a class of chemicals with a variety of medical properties.
The Grignard reaction is an organic chemistry staple. Performing this reaction in the chemistry lab, however, is stressful. The reaction must be performed with minimal exposure to water or air, as both can react with the Grignard reagent. This makes it unforgiving, as its margin of error is rather low (my first attempt and Julia’s first attempt at Reed both failed). The Barbier reaction, named after Grignard’s mentor, is a green alternative that is much easier to carry out. It uses water as the main solvent and is performed in one-pot, making it a worthy alternative.
 The crude reaction mixture of the Barbier allylation of benzaldehyde.
The crude reaction mixture of the Barbier allylation of benzaldehyde.
The oxidation of benzoin to benzil using bleach proved to be my favorite, the most frustrating yet the most rewarding. I had to repeat the experiment five times, tweaking it as I went along, and getting completely different looking products that were actually identical in structure (albeit with some impurities). Simply adding bleach like in my very first experiment was not enough; benzoin is not water soluble, while bleach is. A phase-transfer catalyst (PTC) is required to make the reaction work. A PTC is a chemical, usually a quaternary amine (used in cafeteria disinfectants), that acts as a vehicle, transporting the bleach into the organic layer. Although quaternary amines are widely used as PTC’s in green chemistry, they are biologically hazardous, and thus they are aquatic pollutants. In its place, soy lecithin (bought from my local GNC) was used as an emulsifier. Soy lecithin is edible and its waste is non-toxic (plus it is much cheaper to buy). To my knowledge, its use as a PTC is novel, and has me excited to pursue its applicability further.

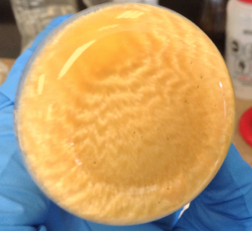
 The many faces of benzil; the wonderful crystals at the top were produced using soy lecithin as a PTC.
The many faces of benzil; the wonderful crystals at the top were produced using soy lecithin as a PTC.
 The product above (white crumbly solid) is adipic acid, produced from the oxidation of cyclohexene. Cyclohexene undergoes through several oxidation steps, among them, several Baeyer-Villiger oxidations. It uses hydrogen peroxide as the oxidizer and sodium tungstate as the catalyst. Best of all, the waste produced is not necessarily waste at all: the aqueous layer can be reused with good results, further increasing its green factor.
The product above (white crumbly solid) is adipic acid, produced from the oxidation of cyclohexene. Cyclohexene undergoes through several oxidation steps, among them, several Baeyer-Villiger oxidations. It uses hydrogen peroxide as the oxidizer and sodium tungstate as the catalyst. Best of all, the waste produced is not necessarily waste at all: the aqueous layer can be reused with good results, further increasing its green factor.
Writing in my lab notebook at my leisure seems slightly out of place now. I miss the hustle and bustle of the lab, of working for hours at a time trying to synthesize a product in a new way, not knowing the protocol beforehand yet not being worried about it. The modest lab I worked in made me comfortable as a chemist. With Reed’s lab and my thesis year around the corner, I am itching to dive even deeper into green chemistry. I’m eager to get the chance to report my findings, and replicate them at Reed where I can begin to think about publishing some of the novel experiments.
Many thanks to Cindy for her help and for letting me spend the summer in the lab, and for spending the days with me!
Tags: psf, presidents summer fellowship, organic chemistry, chemistry
