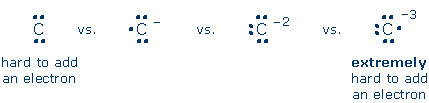
|
|

|
Lewis' Theory of Chemical Bonding Transfer or share? Energy decides Students sometimes fall into the trap of treating Lewis' theory as a "magic number" game: 2 electrons/bond, 8 electrons/atom. Although the theory makes numerical predictions, one should never lose sight of the fact that the "magic numbers" are guided by fundamental physics. Atoms engage in electron transfer and electron sharing only because it lowers their energy. Consider NaCl. Lewis' bonding rules seem to suggest that Na "wants" to make Na+, and Cl "wants" to make Cl-, in order to give each atom a Lewis octet. The truth, however, is that electron transfer from Na to Cl costs energy. The salt forms only because the energy penalty of electron transfer is overwhelmed by the energy benefit of a stabilizing electrostatic attraction between Na+ and Cl-. Lewis' rules also seem to say that compounds like CNa4 are stable because Na+ and C-4 contain Lewis octets. Once again, though, electron transfer is energetically unfavorable. It is energetically unfavorable to transfer an electron from Na to C. Even worse, the energy penalty steadily grows as we attempt to transfer an electron from Na to C-, from Na to C-2, and finally from Na to C-3. The reason for this is simple: a negatively charged electron is repelled by increasingly negatively charged C ions.
To summarize, Lewis' magic numbers are tools for predicting chemical behavior, but they do not have the status of physical principles. Chemical changes are guided by energy and any physical forces, like electrostatic attraction/repulsion, that affect energy. |