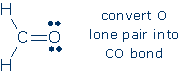
|
|

|
Drawing
Lewis Structures I
This essay describes a drawing method that organic chemists rarely use. Despite this, it appears in nearly every introductory chemistry textbook. Read this essay if you have no idea how to draw a Lewis structure, or if you feel you've forgotten more than you remember.
Drawing rules A Lewis structure is a drawing that shows all of a molecule's valence electrons and all non-zero formal charges. Drawing styles vary from chemist to chemist, but most chemists draw covalent bonds as lines, and nonbonding electrons as dots. No symbols are used for ionic bonds (electrostatic attractions and repulsions are implied by the positions of non-zero formal charges).
A simple drawing method A relatively surefire step-by-step method:
An example: formaldehyde, H2CO 1. Formaldehyde contains 12 valence electrons (2 from 2 H, 4 from C, and 6 from O). 2. C neighbors the other atoms, so draw one bond from C to each of the remaining atoms:
3. The drawing contains only 6 electrons (3 bonds), so 6 electrons are leftover. O is the only terminal atom that lacks an octet, so we add all 6 electrons to O as lone pairs:
Although the rules don't call for it, I've added formal charges to this intermediate drawing. 4. C is the only atom lacking an octet, and O is the only C neighbor that possesses a lone pair. Therefore, we convert an O lone pair into an additional CO bond.
5. Formal charge calculation. Formal charges show whether a Lewis structure assigns too few or too many electrons to an atom. They are called "formal" because they are derived using rules that may, or may not, reflect an atom's true electrical properties. Formal charges are calculated by considering the number of electrons "held" by an atom at any given moment, and not the total number of electrons "seen" by the atom. The "held-not-seen" distinction relates only to bonding electrons: an atom "sees" all of its bonding electrons, but it only "holds" half of them at any given moment. These considerations lead to the following formula for formal charge (FC): FC = #e(isolated atom) - #e(lone pair) - #bonds Let's see how this formula applies to these structures:
The hydrogens are not charged in either formula because an isolated hydrogen atom holds 1 electron and the hydrogens in these formulas hold 1 bonding electron each. C and O are not charged in the formula on the right. C holds 1 electron from each bond; FC(C) = 4 isolated atom - 0 nonbonding - 4 bonding = 0. O holds 4 nonbonding electrons and 2 bonding electrons; FC(O) = 6 isolated atom - 4 nonbonding - 2 bonding = 0. C and O carry opposite charges in the formula on the left. C holds only 3 bonding electrons; FC(C) = 4 - 0 - 3 = +1. O holds 6 nonbonding electrons and 1 bonding electron; FC(O) = 6 - 6 - 1 = -1.
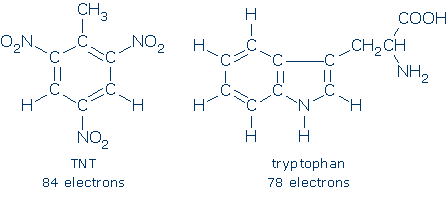
(Im)practicality Organic chemists rarely use the drawing method on this page because it is too slow. Even small organic molecules contain large numbers of electrons (see below) so any drawing method that requires us to count, and recount, electrons is impractical.
|