 Pheromones in Mice
Pheromones in Mice
Biology 342 Fall 2007
Written by: Emmeline Chuu and My Linh Nguyen

Reed College
3203 SE Woodstock Blvd,
Portland, OR, 97202
nguyenm@reed.edu
chuue@reed.edu
Last Modified November 2007
Phylogeny
The study of phylogeny examines the origins and evolution of a set of species to determine ancestral relatedness. Here, we discuss the phylogenetic history of chemical cueing of behaviors across species to better understand the development of the VNO pathway.
Two Main Olfactory Systems
There are two main olfactory systems in vertebrates, the main olfactory system (MOS) and the vomeronasal system (VNO). MOS is receptive to volatile cues, whereas the VNO is receptive to nonvolatile pheromones. The evolution of the VNO has sparked much debate, as it is a sensory organ present in most vertebrates but does not seem to be present in primates or humans [Liman & Innan, 2003].VNO Dependence and Independence between Vertebrates
Further, it has also been discovered that lordosis in female pigs and suckling behaviors in newborn rabbits are unaffected by removal of VNO. However, rodents and mice behaviors are notably changed after removal of VNO. This dichotomous processing of pheromones displays a divergence amongst mammals, as some species process pheromone detection in the MOS and the VNO, whereas other species rely heavily on the VNO for pheromone detection [Leypold et al., 2002].VNO in Aquatic Species was Emergent and Advantageous in Terrestial Species
One of the earliest theories of VNO development posited that the organ developed as an adaptation to terrestrial life [Dulac & Torello, 2003], however, studies soon identified VNO pathways in aquatic species and may indicate that the VNO developed in aquatic species and proved to be more advantageous to terrestrial species [Dulac & Torello, 2003]. Current studies done with TRPC2 (a gene that is required for VNO function in mice and may play a role in the pheromone signaling cascade) may indicate that the selective pressure for terrestrial species to maintain the VNO may have relaxed during primate evolution [Liman & Innan, 2003].
Evolution of the TRPC2 Gene (a gene responsible for VNO function)
It is currently understood that the VNO in primates may have last been functional in the common ancestor of New World and Old World monkeys and apes. Further, it has been proposed that during primate evolution, other signaling pathways, such as the development of color vision, may have relaxed selective pressure on the pheromone- signaling pathway in the VNO [Liman & Innan, 2003].Mutation of the TRPC2 gene began in common ancestor of Old World monkey
Using PCR (polymerase chain reaction) amplification and gene sequencing techniques, Liman and Innan discovered that the TRPC2 gene (a gene that expresses a cation channel which is critical to VNO function in mice) in humans contains six deleterious mutations that generate stop codons, two indels (insertion/deletion genetic mutations), and four missense mutations. Further, these procedures were used on the homologous TRPC2 gene in 15 extant primates, uncovering several frameshift and non-sense mutations in Old World monkeys, leading to truncation of the C terminus and mutations located in the N terminus. In New World monkeys, however, there were no mutations found, suggesting that deleterious mutations in TRPC2 gene began in the common ancestor of Old World monkeys.
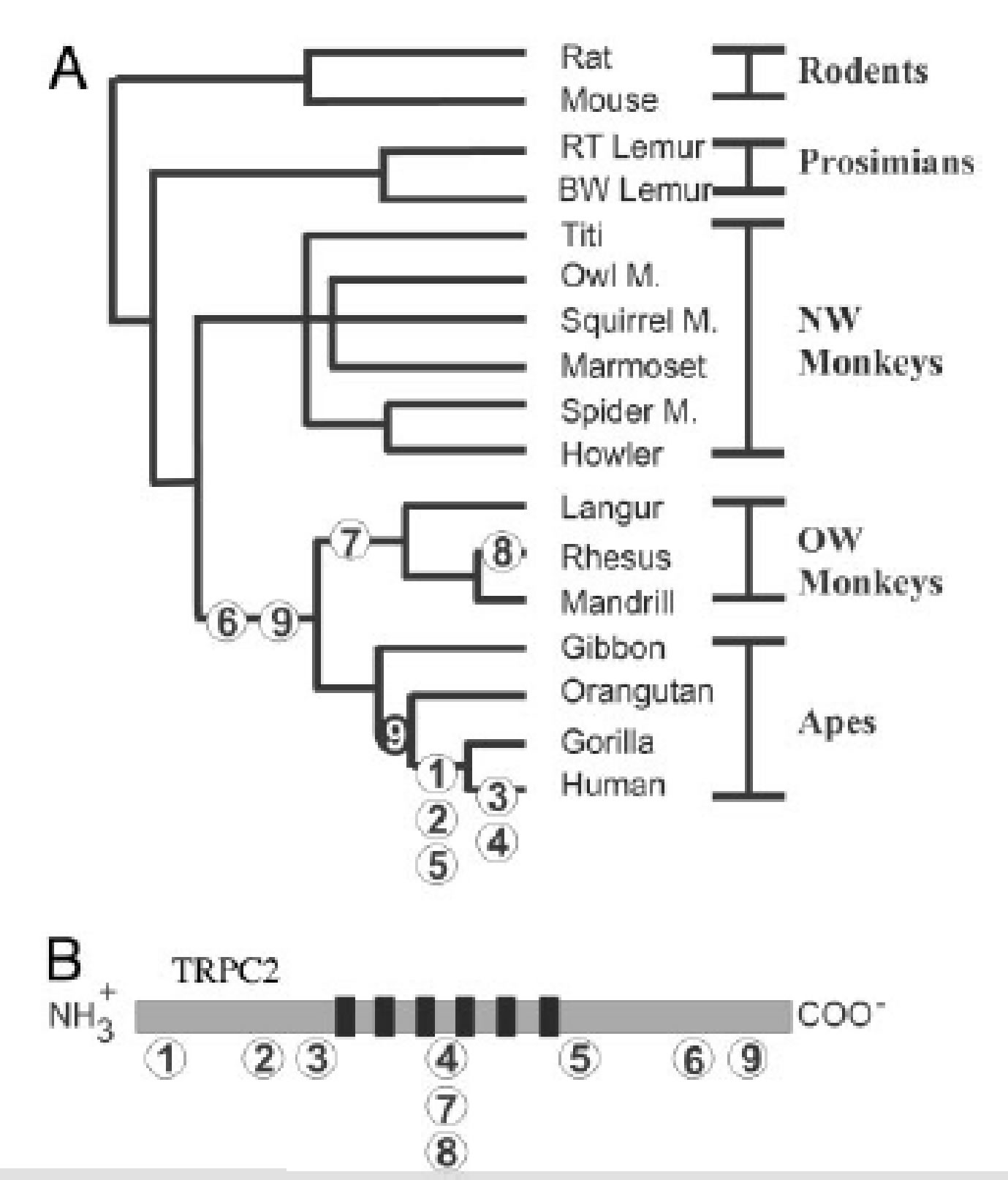
Figure A-B. [Liman & Innan, 2003]
A: Mutations numbered 1-9 in phylogenetic tree that infers time of deleterious mutations in TRPC2 gene.
B: Representation of the TRPC2 ion channel with number markers indicating the position of each mutation. Numbers correlate with mutations in A. Black bars represent transmembrane domains.
Evolution of the V1R Gene (a pheromone receptor gene)
V1R and V2R are two pheromone receptor families that are present in the VNO and know to affect VNO function. Studies have shown a divergence in these receptors in six mammals: the mouse, rat, human, cow, dog, and opossum. Although humans and rodents may be closely linked genetically, the pheromone receptor expressions differ greatly between species [Grus, W.E. et al, 2005].Loss of V1R ancestral genes due to pseudogenes
For example, an analysis of the functional V1R and V2R genes within the six mammalian families found that placental V1R genes had excessive loss of ancestral genes. The loss is due to high number of pseudogenes (genes that are not actively expressed in cells). There are no V2R genes found in non-rodent families. Rodent families such as mice have 187 functional V1R genes and rat have 102 functional V1R genes. Further, cows only have 32 functional V1R genes, dogs have 8, and humans only have 4 [Grus, W.E. et al, 2005].
The loss of V1R gene and VNO function are compensated
These discoveries may indicate that the numbers of V1R genes correlate to the complexity of VNO morphology and the evolution of this gene from rodent to mammals. Again, it is suggested that the loss of VNO function in primates may have been mediated by the acquisition of other abilities, such as the development of tri-chromatic vision [Grus, W.E. et al, 2005]. It has also been suggested that this variability may allow different species to perceive and respond differently to the same chemosensory stimulus to help maintain an effective species barrier [Rodriguez, I., 2005].
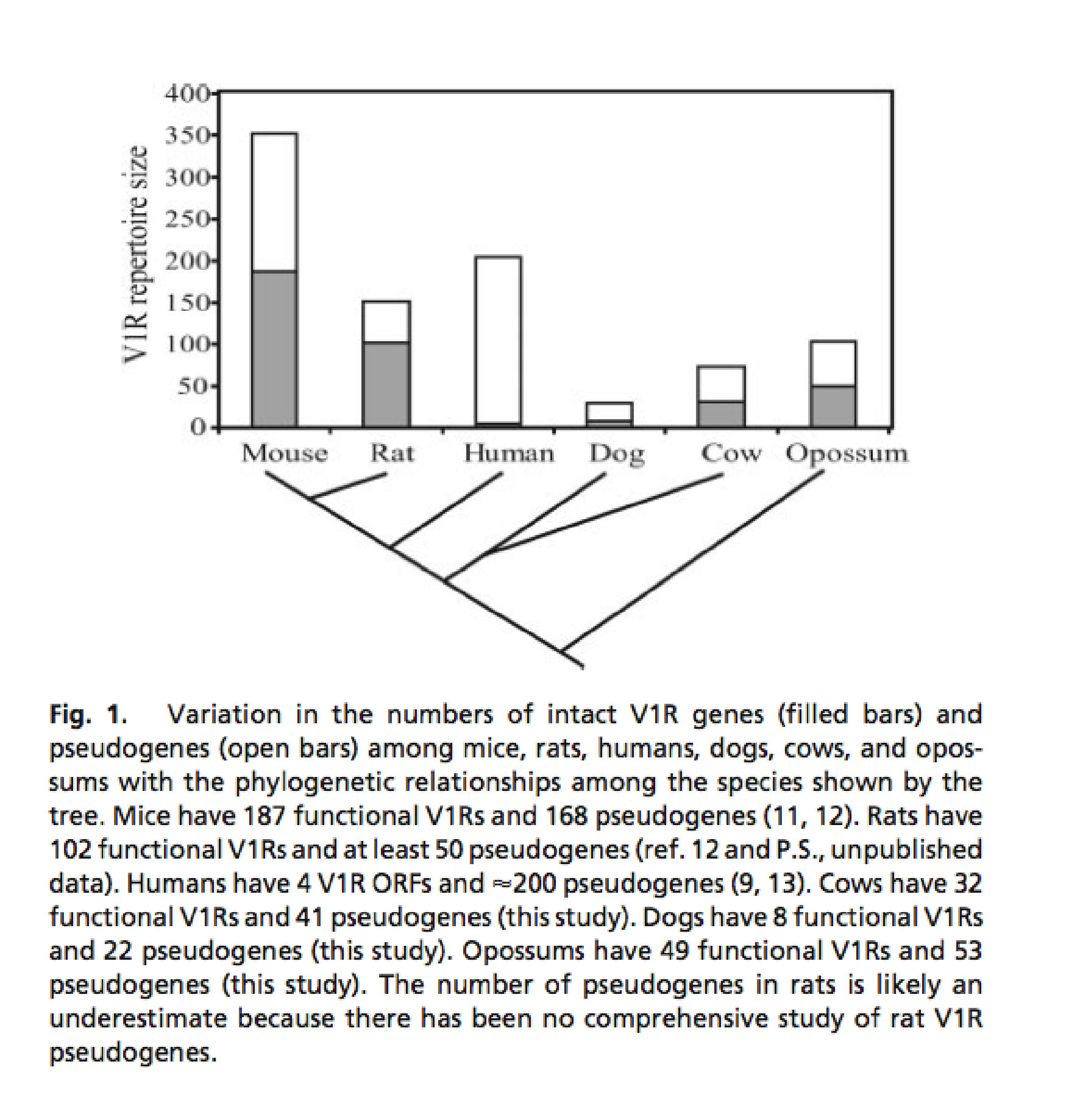
This phylogenetic tree displays the variation in V1R genes throughout different mammals [Grus et al., 2005].