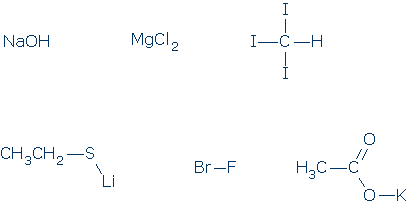
|
|

|
Lewis' Theory of Chemical Bonding Ions and Ionic Bonds Charged particles (or ions) exert electrostatic (or Coulomb) forces on each other. Oppositely charged ions create attractive forces and these create ionic bonds. Ionic bonds are electrostatic whether the ions are atomic, like sodium Na+ and fluoride F-, or polyatomic, like ammonium NH4+ and sulfate SO4-2. Ionic bonds lead to the formation of neutral compounds called salts. Ionic bonds do not change the interacting ions in any major way, so ions maintain their separate charges inside the salt, and are usually discharged as ions when the salt breaks down. For example, sodium sulfate, Na2SO4, dissolves in water by releasing Na+ and SO4-2 ions. Lewis' theory helps us identify atoms that can easily form ions. Ions that carry small charges and maintain inert gas electron configurations should form most easily. These requirements are met by the alkali cations (Li+, Na+, K+), alkaline earth dications (Mg+2, Ca+2), and halide anions (F-, Cl-, Br-, I-). Metals and non-metals nearly always form ionic compounds when they combine. The metals lose electrons and the non-metals gain electrons. Whenever you encounter a formula that combines metals with non-metals, such as Na2O, you should assume that the compound is ionic and you should assign appropriate charges to each component (Na+ and O-2).
Review problems #1. Identify any atoms on the following list that routinely exist as stable ions. What are the charges on these ions? Mg, P, C, H, Cl, B, K, Na, Br
#2. Which of the following compounds are really salts? Try to redraw these formulas to show their ionic components.
|