|

|
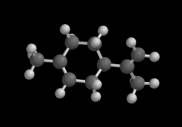
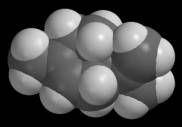
Chapter 2 - Distances Space-filling models Van der Waals radii are used to construct a special kind of molecular model called a space-filling model. These models are constructed by drawing each atom as a van der Waals sphere with the atom’s nucleus at the center of the sphere. Space-filling models are useful because they show how much space an atom (or molecule) occupies. You can see this easily by comparing space-filling models with traditional ball-and-stick models like those in the following figure. The latter do not provide anything like a realistic sense of molecular size.
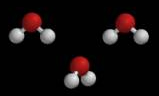
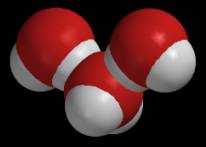
On the other hand, you can also see that it is much harder to establish bond patterns in a space-filling model. Bonds can be located if you recall that bond distances are much smaller than nonbonded distances. A bond must exist between any two atoms that create strongly overlapping spheres. I mentioned above that van der Waals radii are also used to assess nonbonded interactions. The same kind of assessment can be accomplished using space-filling models. The visual counterpart of a distance “prediction gap” is a model that contains two overlapping nonbonded atoms. The following figure compares ball-and-stick and space-filling models of three water molecules. The molecules have been positioned so that they occupy roughly the same positions that they might occupy in an ice crystal.
The ball-and-stick model does not reveal much, but the space-filling model clearly shows that the central oxygen atom substantially overlaps with a hydrogen atom in another water molecule. Distance measurements confirm that the nonbonded OH distance (1.8 Å) is much shorter than the sum of the van der Waals radii (1.2 + 1.4 = 2.6 Å). These observations suggest that water molecules strongly attract each other. This conclusion is supported by some of water’s other strange properties, and chemists have decided that this attractive force deserves a special name. They call it a hydrogen bond. Some scientists claim that water’s hydrogen bonds are responsible for life itself.
|