|
|

|
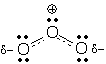
Dashed-line formulas Dashed-line formulas are a tool for drawing resonance hybrids. These formulas differ from normal Lewis structures in two ways: 1) dashed lines are used to show partial bonds, and 2) d- and d+ are used to show partial charges (d is the Greek letter "delta" and is commonly used in science and mathematics to indicate a fractional or partial quantity). Ozone's dashed-line formula shows partial bonds and partial charges:
A dashed-line formula is useful because it tells you right away, "this molecule is a resonance hybrid." It also shows you what parts of the electron pattern are delocalized. Despite these advantages, organic chemists try to avoid dashed-line formulas because they hide necessary information. (Look again at ozone's dashed-line formula. This molecule contains 18 electrons, but can you easily find them? Can you find any atoms with Lewis octets?)
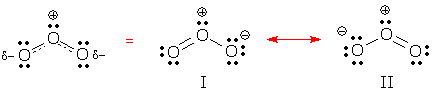
Drawing tips Dashed-line formulas may be hard to read, but they are easy to draw, especially if you have good drawings of the important resonance contributors.
Refer to all of the important contributors, major and minor, and draw:
Notice that delocalized lone pairs are not included in dashed-line formulas. Their absence helps explain why these formulas are hard to use for electron counting. Also notice that special symbols are used only for delocalized bonds and charges. Standard Lewis structure symbols are used for the rest of the drawing. |