|
|

|
Identify a Molecule's Most Basic Atom A methodical approach works best, but there are two different approaches that you can try out.
Method 1 - Strongest base has the weakest conjugate acid First, scan the molecule for all non-halogen atoms with lone pairs (usually N and O). Second, imagine protonating each candidate atom and draw its conjugate acid. Third, identify the weakest conjugate acid. The protonated atom in the weakest conjugate acid is the most basic atom in the original molecule. An example will help you sort through these steps:
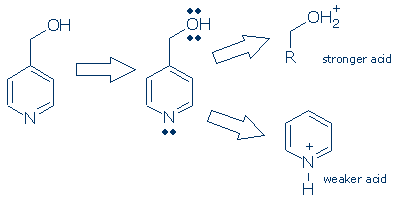
Scanning the molecule for atoms with lone pairs, produces two candidates: the ring N and the O. Next, we draw the conjugate acid of each group. (I have drawn only the acidic functional group in order to save space.) Finally, we evaluate the relative acidity of these imaginary conjugate acids. Pyridinium cation or pyrH+ (pKa +5) is a much weaker acid than ROH2+ (pKa -2), so N is the more basic atom. When the original molecule reacts with an acid, protons will be transferred to N selectively because they will be less able to leave this atom. This method may look a little convoluted, but it becomes quite easy after a little practice.
Method 2 - Scan a molecule for strongest known basic group Modern organic chemists tend to focus on acid strength. When we need to think about base strength, we remember the old saying, "the conjugate acid of a strong base is weak, and vice versa". This is why I first showed you how to find the most basic atom by looking at various conjugate acids. However, you can also learn to recognize basic groups and learn their relative reactivities. If you have this information at your fingertips, you can scan a molecule directly for these groups. Referring to the molecule above, I know that pyridine (C6H5N) is a stronger base than water (which I consider a type of alcohol), so N is the most basic atom in this molecule. If you can't remember the properties of common basic groups, it is also possible to fall back on some general principles: #1 Importance - negatively charged bases are stronger than neutral bases. Positively charged molecules are rarely basic. #2 Importance - look for deactivating groups, including RSO2, RC=O, and Ph. These groups deactivate a base. #3 Importance - all things being equal, an N base is stronger than an O base. #4 Importance - within a functional group category, use substituent effects to compare bases. Each of these principles is accompanied by important restrictions. These are explained in the essay, Identify a Molecule's Most Acidic H, and you should turn there for a detailed explanation. Remember as you read that essay, that whatever makes a compound a stronger acid will weaken its conjugate base, and vice versa.
Review problems under construction |