Acoustic Mimicry
Biology 342 Fall 08
Phylogeny
Phylogeny, from the Greek phyle = tribe, race and genesis = birth, refers to the evolutionary relationships between organisms. The phylogeny of a specific organism refers to its evolutionary history or lineage. Phylogenies can be reconstructed in a variety of ways. Some are based on molecular similarities between groups of organisms, others are based on morphological comparisons gleaned from the fossil record, and still others use combinations of the both approaches. It is often interesting to know whether a specific trait has arisen multiple times in organisms that are distantly related or whether they have arisen only once and all organism with that trait are descendent form a common ancestor.
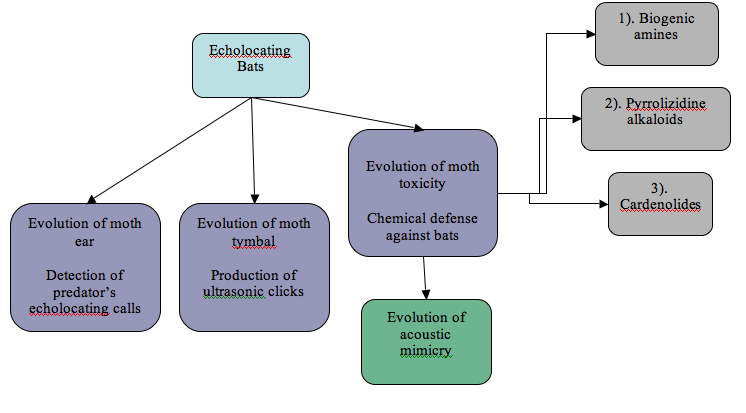
Flowchart demonstrating the evolution of different sectors in the bat-moth predator-prey paradigm involving acoustic mimicry (each have been explained in the text below)
Evolution of mimicry
In a complex system where multiple prey species are available, there is an intense selection on unprofitable prey to mimic each other (Beatty et al., 2004). The primary selective force is acquired learning in predators enabling them to better distinguish between profitable and unprofitable preys based on the distinct phenotypes of the latter (Beatty et al., 2004; MacDougall and Dawkins, 1998; Ruxton, 1998). Unlike Muller’s suggestion, predators do not completely rely on associative learning while foraging but rather use simple rules for discriminating between profitable and unprofitable preys (Beatty et al, 2004). Mullerian mimicry is more likely to arise in a multispecies community such as that observed in the bat-moth interaction paradigm.
There have been conflicting theories posited on the evolution of mimicry as being sudden versus gradual (Rettenmeyer, 1970). One side of the argument propounds that for effective protection against predators, organisms must evolve mimicry via major mutations or saltations as envisioned by Goldschmidt (1945 and 1952). On the other side, it has been demonstrated using mimic-model pairs that mimetic evolution is a gradual process, an accumulation of small steps over a period of time. Fisher argued in 1958 that a mimic arising from a macromutation in a switch gene would be comparable of females arising from males since sex is determined by a single chromosome or a few genes. It is more plausible that mimics in a predator-prey paradigm like ours arose from gradual evolution over a long period of time under the predator-induced selective pressures.
Selection for or against aposematic strategies like acoustic mimicry is susceptible to variation in predator and prey that engender variation in profitability among species and individuals. The net profitability of preys will vary in the lifetime of an individual predator which can be attributed to the relative abundance of other preys, fluctuation in the nutritional value of the prey, efficacy of its defense mechanism, etc. Aposematism in general and acoustic mimicry in particular can be affected by variations in selection induced by microgeographical and geographical factors (Mappes et al, 2005):
- A predator in a poor territory or marginal habitat characterized by a dearth of alternate prey might be encouraged to feed on aposematic species more so than in average territories, given that there aren’t substantially detrimental effects.
- Cultural transmission and social interactions could influence feeding behavior giving rise to localized feeding cultures (Altbaecker V et al., 1995). Socially induced preferences for food have been studied in fruit bats in which the bats learned about novel foods through roosting interactions with other conspecifics, and these socially learned preferences were seen to be reversible by further social interaction (Ratcliffe and Hofstede, 2005).
- A predator could colonize a new territory giving rise to a new aposematic selection pressure on the local prey population and aposematic preys could colonize new areas presenting a novel challenge to the local predators (Phillips BL and Shine R, 2004)
- Clumped prey distribution also plays an important role in affecting selection for aposematism (Ruxton GD, 2004). In a bad year or a low-quality territory where prey fauna is minimal, clumped prey distribution will select against aposematism since predators will be more likely to stay in the habitat patch that has a good prey population irrespective of aposematic aspects. On the other hand, being in a clumped distribution in a prey-rich territory/season would select for aposematism since predators would choose alternate preys over aposematic ones
The origin, spread, and maintenance or the evolution of aposematism is a therefore a function of variations in space and time. In a good year or in a high-quality territory teeming with prey fauna, the predator might be highly selective, avoiding even the weakly aposematic preys which would enable the initial spread and further strengthening of aposematism in these prey populations. We would expect these new forms or weakly aposematic preys to be a developmentally plastic population whose aposematism would get entrenched in the population by genetic accommodation (West-Eberhard, 2005) and further enhanced given the maintenance of stable conditions of avoidance by the predator. An intermediate season/territory would select for the existing aposematism but not support novel, burgeoning ones still in their weak primitive stages. In low-quality territories or in a bad year, aposematism will be selected against since starving predators wouldn’t discriminate between aposematic and alternate preys unless aposematism has evolved for so long in these territories that it has developed to incorporate emetic or predator-weakening effects.
Evolution of echolocation in bats
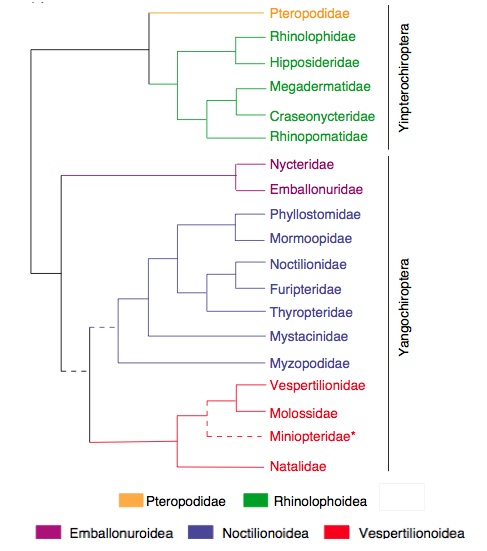
The evolution of raptorial or predaceous birds has been suggested to drive the bats into being nocturnal predators leading to the evolution of specialized abilites: echolocation in microchiropterans and nocturnal vision in megachiropterans (Speakman, 2001). Recent molecular phylogenies suggest that laryngeal echolocation (that is, echolocation calls produced in the larynx) evolved in the ancestor of all existing bats, only to be lost in the present day non-echolocating bats (Pteropodidae: Old World fruit bats). Different acoustic phenomena (like nasal emission of sound in some bats) and the wide range of bat calls indicating adaptive radiation (one species giving rise to others in response to different niches) and convergent evolution (different species exhibiting similar features due to adaptation to similar environments) lends credence to the fact that echolocation is plastic and evolves in accordance to the demands of ecology (Jones and Teeling, 2006).
Molecular tree of existing bat families constituting of two clades: Yinpterochiroptera and Yangochiroptera. The latter clade consititutes of all echolocating bats while the former clade includes the non-echolocating (except by tongue-clicking in one genus) superfamily Pteropodidea with the echolocating superfamily Rhinolophoidea. Colored boxes, branches and names represent the six superfamilial groupings shown. (Jones and Teeling, 2006)
Evolution of moth ear
The metathoracic ear of tympanal moths that are used to detect the echoloating calls of bats is believed to have evolved from the mesothoracic wing-hinge chordotonal organ. Based on its association with a more primitive, unspecialized structure (atympanate wing membrane), this chordotonal organ is considered to be representative of a pretymapanal condition where moths hadn’t developed the capacity of hearing bats. This evolution from the pretympanal to tympanal state included neural modifications that have been key in relaying information about the bat’s echolocating calls to the moth’s central nervous system and for governing the evasive flight maneuvers adopted by the moths upon detection of the nocturnal predator. 
Evolution of tymbal:
The tymbals of sound-producing arctiids differ from the unmodified episterna (one of the lateral pieces next to the sternum, which is the ventral portion of a segment in the thorax) of silent moth species in their exoskeletal structures and the size of the air sacs (used as sound amplification devices); interestingly, asides from these peripheral differences, substantial differences have not been observed in the neuromuscular functioning (Fullard and Heller, 1990). Based on these observations, it is concluded that the arctiid tymbal arose from the episterna of silent Lepidoterans (moths of the order to which the arctiids belong) with only minor modifications.
Evolution of toxicity
It has been argued that in response to an intense selection pressure from predators like bats towards chemically protected tiger moths, these arctiids have been sequestering greater quantities of the natural deterrants (Histrov and Conner, 2005). Based on phylogenetic evidence, a general trend has been observed in the evolution of chemical defense in the arctiids in which the least effective biogenic amines have been posited to be the primitive trait of the arctiids. The moth’s ability to sequester the more effective pyrrolizidine alkaloids came later finally followed by the procuring of the most efficacious cardenolides (Weller et al., 1999). The inclusion of iridoid glycoside into the chemical arsenal of the arctiids hasn’t as of yet been clear. This later inclusion of the more effective pyrrolizidine alkaloids and cardenolides into the chemical defense mechanism of the moths demonstrates a stronger arms race or a more intense selection pressure from the predators.
Sequential demonstration of approach and capture of a non-toxic moth (Galleria mellonella) by a big brown bat (Eptesicus fuscus); the sequence delineates multiple points where the decision to reject the prey (if unprofitable) can be made. (Hristov and Connor, 2005)