Acoustic Mimicry
Biology 342 Fall 08
Mechanism
Mechanism or causation is a proximate description of an organism’s structures and how they function. It refers to the proximate causes (such as genes, hormones, and nerve impulses) of a behavior that control its expression. In order gain a deeper understanding of the mechanism of acoustic mimicry, we describe the structures involved in this mimicry paradigm – the chemical defense of toxicity, the warning sound producing tymbals, and the bat echolocation call detecting ears. (Source: Suzy Renn's course wesbite)
Toxicity
Tiger moth’s status as an unprofitable prey is due to the allelochemics obtained by the moths from the their larval food. The most prevalent of these chemical defenses are biogenic amines biogenic amines (BAs), iridoid glycosides (IGs), pyrrolizidine alkaloids (PAs) and cardiac glycosides (CGs) (Weller et al. 1999; Nishida 2002). Palatability of the moth has been reported to be highly dependent on the moth’s diet giving rise to a range of toxicity – feeding on plants containing cardiac glycoside and pyrrolizidine alkaloid results in greater noxiousness towards bats than feeding on biogenic amines or iridoid glycosides (Hristov and Conner, 2005b).
These allelochemics can be sequestered by both the adult and the larvae and can be transmitted between sexes and transovariantly from mother to offspring. Once sequestered, these defense chemicals are transmitted to the locations that would be susceptible to a predator attack: eggs, larval integument and hemolymph, cocoon, and in adult integument, scales, hemolymph and reproductive structures. The table below enlists the source of the procured deterrents ranking them in order of defense efficacy.
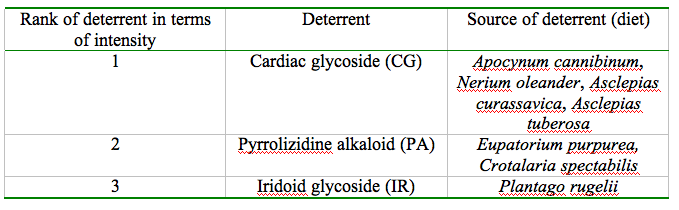
Even though the highest degree of unpalatibility is attributed to CG, variance in the noxiousness of moths raised on CG-laden diet has been observed. While Cycnia tenera and Empyreuma affinis procure CG from their larval food (Black, 1976; Cohen and Brower, 1983) and have been successfully in repelling bats, Euchaetes egle, a Batesian mimic of the ultrasonic click producing tiger moths, which also feeds on CG is found to be a palatable prey for the bats. Despite the fact that the hostplants of the highly noxious C.tenera and the palatable E.egle have been found growing in the same fields with the two moths resting on each other's hostplants (Barber and Conner, 2007), the difference in palatability between these two species is striking. It has been concluded that E. egle could be metabolizing or excreting most of the CG ingested to achieve this comparative deficiency of unpalatability (Hristov and Conner, 2005b).
Tymba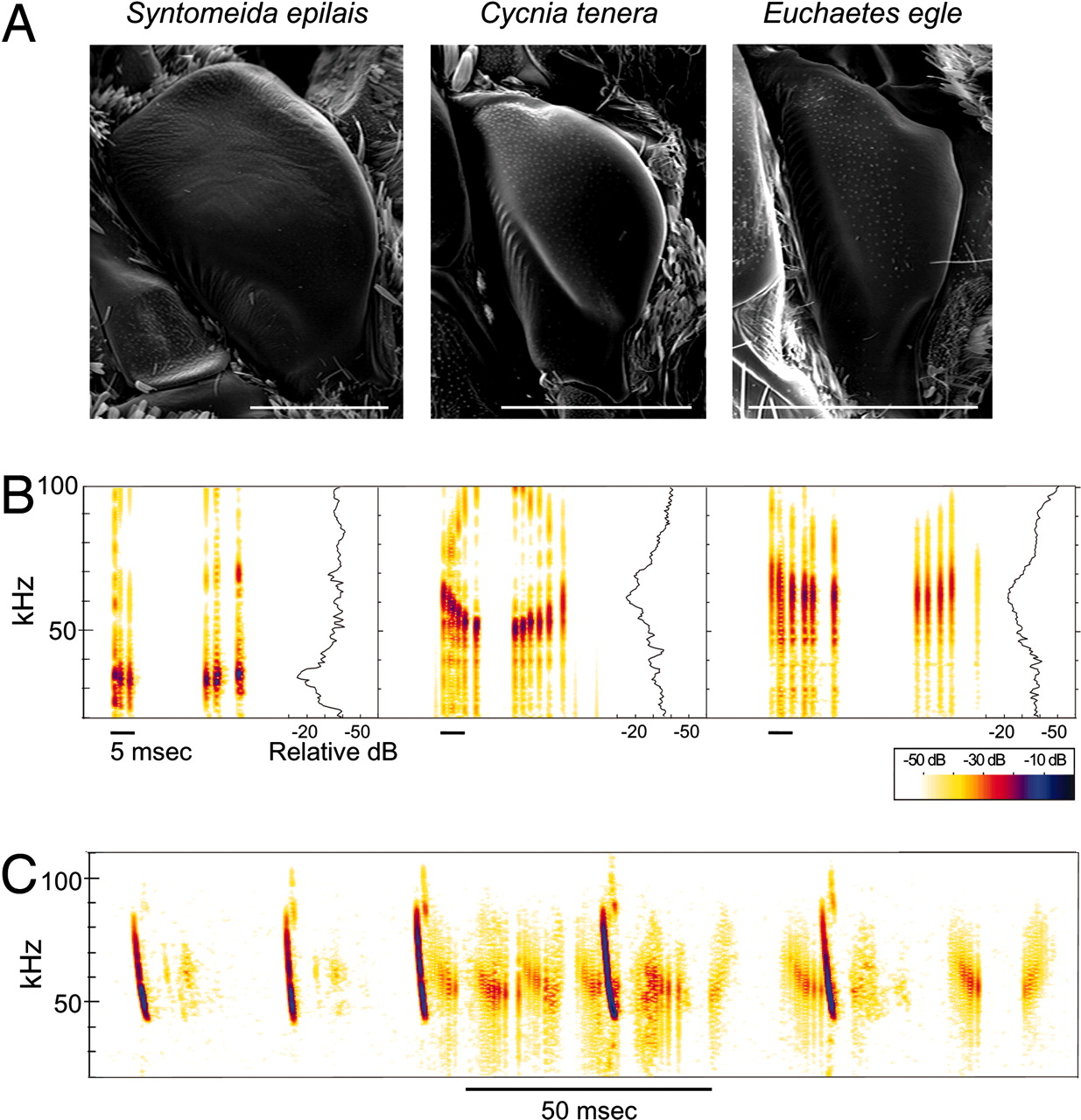 l
l
The remarkable feature of ultrasonic sound production in the arctiid moths occurs in the metepisternal tymbal organs (Simmons and Conner, 1996). These well-developed metathoracic structures function primarily in defense against bats, producing aposematic ultrasonic clicks that jams the echolocation signals of the predator (Fullard et al. 1979; Miller, 1991) or warns the bats of the underlying chemical protection (Simmons and Conner, 1996; Dunning et al. 1992). In the arctiid Mullerian (Syntomeida epilais) and Batesian mimics (Euchaetes egle) of the noxious tiger moths (Cycnia tenera), the paired tymbals engage in acoustic mimicry sending out honest and dishonest signals of unpalatability respectively.
In these clever mimics and their chemically armored models, the anterior outer edge of the tymbal is a striated band replete with ridges or microtymbals. The inward buckling (flexion) of this striated band produces an initial burst of clicks, each emanating from a single microtymbal. A second burst of sound is then produced by the passive recoiling of the tymbal to its original shape (relaxation) (Fullard and Heller, 1990; Figure 1). These two bursts of pulses comprise a single call in these arctiid species. These clicks may operate differently in different species and may also be dependent on age and experience of the bats (Conner, 1999).
Figure 1. Sound production in the arctiid acoustic mimicry complex. (A) Scanning electron micrograph of the metathoracic tymbals showing the outer striated ridge with microtymbals. (B) One complete modulation cycle of the tymbal-produced sound showing two distinct sound bursts produced during flexion (active modulation half-cycle) and relaxation (passive modulation half-cycle) and the intracycle silent phase. (C) Spectrogram of the ultrasonic clicks produced by a C. tenera in response to the echolocation signals of the red bat Lasiurus borealis. The sound production appears to be different from B due to asynchronous activity of the paired tymbals. (Barber and Conner, 2007)
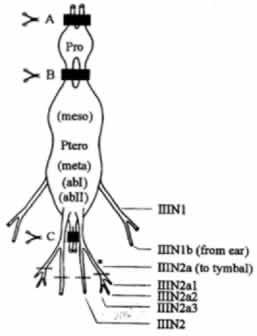
The thoracic central nervous system of arctiid moths constitute of the prothoracic and the pterothoracic ganglion. The latter is a fusion of the mesothoracic, metathoracic, and the first and second abdominal ganglia. The thoracic CNS is innervated by the nerve IIIN1b from the insect ear and gives rise to the metathoracic leg nerve which branches into tymbal nerve, IIIN2a, which innervates all of the tymbal muscles (Fullard and Heller, 1990). This IIIN2a nerve trifurcates into IIIN2a1, IIIN2a2, and IIIN2a3.
The tymbal activity is governed by a central pattern generator (CPG) located within the CNS of the moth. Despite the fact that the minimum circuity sufficient for the functioning of the tymbals resides in the pterothoracic ganglion, the prothoracic and cephalic ganglia are essential for normal and auditory-induced operation of the CPG (Dawson and Fullard, 1995).
The thoracic CNS of C. tenera (Dawson and Fullard, 1995)
Click here to watch C. tenera tymbals in action! (Barber and Conner, 2007)
Moth Ear 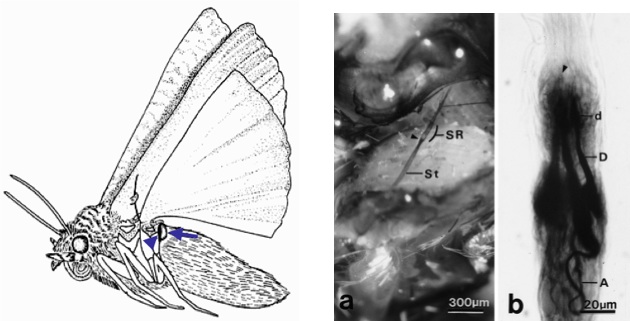
Tympanate moths possess sensitive, highly few-celled ears on either side of the posterior metathorax. Each ear constitutes of a tympanic cavity, which houses the tympanic membrane, and attached to the inner side of this membrane is the chordotonal tympanal organ. This organ contains the bipolar sensory cells, which detect the echolocation calls from insectivorous bats enabling the moths to engage in evasive flight action. Alongside these sensory cells, the chordotonal organ also constitutes of a distal attachment strand that attaches to the metathoracic wing-hinge region as shown in the figures below.
Left: Lateral view of the ultrasonic click producing noctuid moth showing the location of the tympanic cavity (arrowhead), which contains the tympanic membrane.
Right: Light micrographs of the chordotonal organ (a; arrowhead indicating the proximal region of the chordotonal organ called the scolopidial region (SR); St-chordotonal organ strand) and the three bipolar sensory neurons (b; A-axon, D-dendrite, d-dilation of dentrite). (Yack et. al, 1999)
In brevity, the bats send out their echolocating calls (at 20-50 KHz, the lower range of the bat ultrasound) to detect moths, which are then perceived by the moth’s metathoracic ears. As the bat approaches closer to the moth, the loudness and frequency of its echolocating call increases so as to go undetected by the moth ea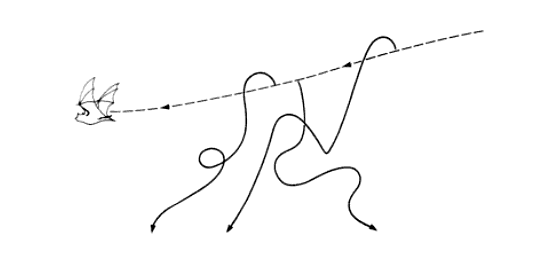 rs at these high frequencies; however, in response to this, the moth too tunes up its ear sensitivity to be now receptive to the higher frequency of the approaching bat (Windmill et. al, 2006). The moth engages in evasive flight maneuvers (as shown in figure below) and its tymbal starts producing ultrasonic clicks (in case of sound-producing moths), which could either be an honest or a dishonest signal of the underlying toxic chemical defense. Toxicity to bats is developed by noxious moths by sequestering toxic chemicals from their larval food sources, a procedure that their non-toxic mimics failed at, which renderd these mimics palatable to the predator.
rs at these high frequencies; however, in response to this, the moth too tunes up its ear sensitivity to be now receptive to the higher frequency of the approaching bat (Windmill et. al, 2006). The moth engages in evasive flight maneuvers (as shown in figure below) and its tymbal starts producing ultrasonic clicks (in case of sound-producing moths), which could either be an honest or a dishonest signal of the underlying toxic chemical defense. Toxicity to bats is developed by noxious moths by sequestering toxic chemicals from their larval food sources, a procedure that their non-toxic mimics failed at, which renderd these mimics palatable to the predator.
Evasive pathways that could be taken by a moth being hunted by a bat (Image courtesy of Behavior and Evolution by Marion Hall)