The Zombie Ant: Parasitic Fungi and Behavior Manipulation
Biology 342 Fall 2014
Kristin Hirata
Phylogeny
There are potentially millions of species in the Fungi kingdom, and some of these species are parasitic. Different fungi can infect a variety of hosts, from plants to insects to mammals. How did O. unilateralis evolve to have the characteristics it has today?
Host specifity and coevolution are two significant factors in the evolutionary history of Ophiocordyceps unilateralis.

Figure 6. Ophiocordyceps sinesis after it has been cleaned and dried. Source: The Rufford Foundation
Over time, various Ophiocordyceps species have evolved, each targeting a specific host.
Researchers have found evidence of the “death grip” on leaves from 48 million years ago in Messel, Germany, indicating that this phenomenon is not one that has recently evolved (Hughes, Wappler and Labandeira, 2011). Other fungus species of the genus Ophiocordyceps have evolved to target different insects, such as varying species of ants, caterpillars, and stinkbugs. One example of this is Ophiocordyceps sinesis, a species that infects caterpillars in China, which is depicted above (Fun fact: O. sinesis, as unpalatable as it may seem, is popular as a Chinese herbal remedy!). One defining aspect of Ophiocordyceps species is that they evolved to have great host specificity; each species normally infects one or possibly two host species at most.
There are many disadvantages associated with host specificity. Normally, a parasite that resides in an area where there are not many hosts will evolve over time to have a greater ability to infect more hosts. If this is the case, then why is Ophiocordyceps unilateralis so host-specific, especially since its host rarely ventures down to the forest floor where the fungus dispenses its spores? Recent research may offer insight into this question. Attempts to infect two naturally infected ant species, Camponotus castaneus and Camponotus americanus, and two species that are not naturally infected, Camponotus pennsylvanicus and Formica dolosa, with Ophiocordyceps unilateralis sensu lato revealed that, while the fungus was able to kill all four species, it was only able to manipulate the behavior of the species that it naturally infects (Bekker et al., 2014). This suggests that the fungus can only manipulate the behavior of one or two specific host species because Ophiocordyceps unilateralis cannot produce the correct combination of compounds to successfully alter behavior of numerous species. The implication is that over evolutionary time, the benefits of altering a hostís behavior and finding a location ideal for fungal proliferation outweighed the costs of having fewer hosts available to infect.
Research tentatively supports this hypothesis. Scientists found that dead ants infected by O. unilateralis in Brazil were located in a wider range of heights than ants infected by O. unilateralis in Thailand. The discovery suggests that in Brazil O. unilateralis evolved to have less fine tuned behavioral manipulation due to a wider range of appropriate growth conditions (Andersen and Hughes, 2012), hence providing provisional support for a relationship between the evolution of the alteration of host behavior and conditions ideal for fungal proliferation.
This phenomenon emphasizes a concept called the extended phenotype, which refers to the fact that the phenotypes of hosts and parasites result from interactions between the two and not simply from one or the other (Lambrechts et al., 2006). The figure below illustrates different forms of parasite-host interactions. In O. unilateralis, the extended phenotype consists of the fungus expressing its genes through the host phenotype (Andersen and Hughes, 2012).
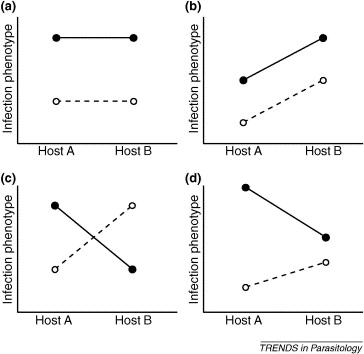
Figure 7. Different types of phenotypic parasite-host interactions. The dark line/dark circles and the dotted line/light circles represent different parasites. Source: Lambrechts et al., 2006
In scenario (a), there is only an effect of the parasite genotype, with no interaction effect from the host genotype. In scenario (b), there is an interaction effect between host and parasite genotypes; host B amplifies the parasiteís infection phenotype. Scenarios (c) and (d) illustrate different types of interaction effects. In (c), one parasite loses potency in host B whereas the other gains potency. Scenario (d) demonstrates a similar trend as (c).
A brief aside, for the sake of accuracy:
Researchers have actually found that O. unilateralis is in itself not a single phylogenetic species. O. unilateralis is, in fact, a species complex comprised of at least three separate species (and possibly hundreds), each specializing in a single formicine ant species (Kobmoo et al., 2012; Evans et al., 2011). However, for the sake of simplicity, in the section above O. unilateralis is referred to as a single species that infects C. leonardi, since O. unilateralis as a whole does predominantly infect C. leonardi.
It is likely that O. unilateralis co-evolved with its host ant, each exerting selective pressures on the other.
Scientists theorize that Ophiocordyceps unilateralis' host ants evolved to avoid the forest floor in order to prevent infection by fungal parasites. In response, O. unilateralis, under selective pressure due to the scarcity of its host in the areas where the fungus dispensed spores, evolved interparous reproduction, releasing a few potent spores occasionally (as opposed to many spores all at one time) in order to maximize its chances of infection. Thus O. unilateralis and its host co-evolved due to selective pressures induced by one another.
Broadly defined, coevolution refers to the process by which the evolutions of two species affect each other. For parasites specifically, the Fahrenholz rule states that parasite phylogeny mirrors host phylogeny. Research in the evolution of different parasites and their hosts provides support for these ideas by demonstrating that parasites and their hosts tend to speciate together, and their respective speciations are probably casually related (Hafler and Nadler, 1988). In this case, it is probable that C. leonardi and O. unilateralis evolved until both were able to survive and successfully reproduce given the pressures exerted by the other species. C. leonardi normally avoid the forest floor, and the ants that are infected are isolated from the colony. Because O. unilateralis is so potent that it can easily wipe out an entire ant colony, both of these host ant characteristics limit the spread of the fungi and enable the overall survival of C. leonardi. On the other hand, O. unilateralis does not infect ants often, but it is able to survive and reproduce by maximizing its chances of finding a host and producing effective spores.